Ethyl bromide experiment
Materials and Instruments
Round Bottom Flask, Erlenmeyer Flask, Hollow Stopper
Step
Instrument: Organic synthesis preparation instrument
2 100 mL round-bottom flasks; 2 150 mL conical flasks; 1 hollow plug; 1 500 mL beaker; 1 vacuum pipe; 75°C elbow; distillation head; 1; thermometer sleeve; 2 separating funnels; measuring cylinder 10 mL; heating mantle 250 mL, dropper.
Medicines: 95% ethanol, concentrated sulfuric acid, solid sodium bromide.
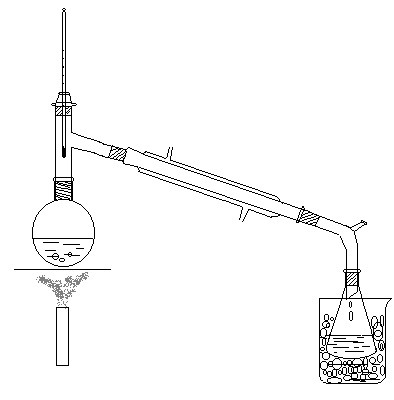
Experimental device:
Add 10 mL of 95% ethanol and 9 mL of water to a 100 mL round-bottomed flask, under constant shaking and cooling with cold water, add 19 mL of concentrated sulfuric acid, cool to room temperature, add 15 g under cooling and grind into fine powder The sodium bromide, after a little shaking and mixing, add a few grains of zeolite, and install it into an atmospheric distillation device (see the device diagram). An ice-water mixture should be placed inside and outside the receiver to prevent volatilization loss of the product. The branch pipe of the liquid pipe is connected to the sewer or outdoor with a rubber pipe. The reaction mixture was heated and distilled on asbestos mesh with low heat, so that the reaction took place smoothly until no oily substance was distilled out.
Pour the distillate into a separatory funnel, place the separated organic layer in a 150 mL dry conical flask, and in an ice-water bath, add concentrated sulfuric acid dropwise while shaking until the sulfuric acid layer is separated from the bottom of the conical flask . Use a dry separatory funnel to separate the sulfuric acid solution, pour the crude bromoethane product into a dry distillation flask, heat and distill in a water bath, cool in an ice-water bath outside the receiver, and collect the fractions at 37-40 °C. Weigh and calculate yield.
Pure bromoethane b.p. 38.4 °C, 1.4239.
Precautions
1. The device should be tight.
2. When adding concentrated sulfuric acid, it should be shaken and cooled while adding, and after sufficient cooling (in an ice-water bath), sodium bromide should be added to prevent the reaction from exothermic flushing out.
3. The heating should be carried out with a small fire first, and then gradually increased, so that the reaction can occur smoothly and avoid large fire, otherwise the product will be lost and by-products will be formed.
4. When refining, the water should be completely removed first, and sulfuric acid should be added under cooling, otherwise the addition of sulfuric acid will generate heat and cause volatilization of the product.
5. Pay attention to drying in the final distillation.
6. Product acceptance quality or volume and refractive index.
Conical Flask 250ml Erlenmeyer,Conical Flask 250ml Manufacturer,Conical Flask 5000ml Manufacturers
Post time: Apr-22-2022